Classes- Alabama
- Alaska
- Arizona
- Arkansas
- California
- Colorado
- Connecticut
- Delaware
- Florida
- Georgia
- Hawaii
- Idaho
- Illinois
- Indiana
- Iowa
- Kansas
- Kentucky
- Louisiana
- Maine
- Maryland
- Massachusetts
- Michigan
- Minnesota
- Mississippi
- Missouri
- Montana
- Nebraska
- Nevada
- New Hampshire
- New Jersey
- New Mexico
- New York
- North Carolina
- North Dakota
- Ohio
- Oklahoma
- Oregon
- Pennsylvania
- Rhode Island
- Tennessee
- Texas
- Utah
- Vermont
- Virginia
- Washington
- West Virginia
- Wisconsin
- Wyoming
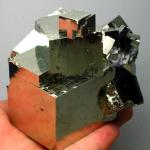
| Structure : Cubic Hardness : 6 Specific Gravity : 4.9 Refractive index : none Doubly Refracting (bi-refraction) : none “Fool's Gold” redirects here. For other uses, see Fool's Gold (disambiguation). Pyrite A mass of intergrown, striated pyrite crystals General Category Mineral Chemical formula iron persulfide (FeS2)Identification Color Pale, dull gold Crystal habit Cubic, faces may be striated, but also frequently octahedral and pyritohedron. Often inter-grown, massive, radiated, granular, globular and stalactitic. Crystal system Isometric; bar 3 2/m Cleavage Poor Fracture Very uneven, sometimes conchoidal Mohs Scale hardness 6 - 6.5 Luster Metallic, glistening Refractive index Opaque Streak Greenish-black to brownish-black; smells of sulfur Specific gravity 4.95 - 5.10 Melting point 1,177-1,188°C [1] Fusibility 2.5-3 Solubility insoluble in water Other Characteristics paramagnetic The mineral pyrite, or iron pyrite, is iron sulfide, FeS2. It has isometric crystals that usually appear as cubes. The cube faces may be striated (parallel lines on crystal surface or cleavage face) as a result of alternation of the cube and pyritohedron faces. Pyrite also frequently occurs as octahedral crystals and as pyritohedra (a dodecahedron with pentagonal faces). It has a slightly uneven and conchoidal fracture, a hardness of 6–6.5, and a specific gravity of 4.95–5.10. It is brittle, meaning it breaks or powders easily. A major identifier, useful in the field, is the streak, as the powdered mineral smells of sulfur. Its metallic luster and pale-to-normal brass-yellow hue have earned it the nickname fool's gold, but ironically, small quantities of actual gold are sometimes found in pyrite. In fact, such auriferous pyrite is a valuable ore of gold. Pyrite is the most common of the sulfide minerals. It is usually found associated with other sulfides or oxides in quartz veins, sedimentary rock and metamorphic rock, as well as in coal beds, and as the replacement mineral in fossils. Euhedral cubic pyrite crystals Pyrite exposed to the environment during mining and excavation reacts with oxygen and water to form sulfuric acid, resulting in acid mine drainage. This results from the action of Thiobacillus bacteria, which generate their energy by using oxygen to oxidize ferrous iron (Fe2+) to ferric iron (Fe3+). The ferric iron in turn reacts with pyrite to produce ferrous iron and sulfuric acid. The ferrous iron is then available for oxidation by the bacteria; this cycle can continue until the pyrite is exhausted. Pyrite is used for the production of sulfur dioxide, e.g. for the paper industry, and in the manufacture of sulfuric acid, though such applications are declining in importance. Pyrites can show negative resistance and have experimentally been used in oscillator circuits as radio detectors. The name pyrite is from the Greek word Pyr meaning fire. This is likely due to the sparks that result when pyrite is struck against steel. This capacity made it popular for use in early firearms such as the wheellock. Cubes of fully or partially oxidized and hydrated pseudomorphs of pyrite are known colloquially as devil's dice. |
- Birth Stone Gems
- Wedding & Anniversary Gems
- How To Guides
- Fireable Gems
- Nonprofits and Guilds
- Gem Show
- Gem Stone Information
- ·Abalone
- ·Achroite (Tourmaline)
- ·Agate ( Chalcedony)
- ·Alexandrite
- ·Almandine ( Garnet )
- ·Amber
- ·Amethyst ( Quartz)
- ·Ammolite
- ·Andalusite
- ·Andradite Garnet
- ·Apatite
- ·Aquamarine ( Beryl )
- ·Aventurine ( Quartz )
- ·Azurite
- ·Benitoite
- ·Bloodstone ( Chalcedony )
- ·Brown Quartz ( Smokey Quartz )
- ·Calcite
- ·Carnelian ( Chalcedony )
- ·Cassiterite
- ·Celestine
- ·Cerussite
- ·Chalcedony
- ·Chatoyant Quartz
- ·Chrysoberyl
- ·Chrysocolla
- ·Chrysoprase (Chalcedony)
- ·Citrine ( Quartz )
- ·Coral
- ·Danburite
- ·Diamond
- ·Diopside
- ·Dioptase
- ·Dravite ( Tourmaline)
- ·Emerald ( Beryl )
- ·Enstatite
- ·Epidote
- ·Euclase
- ·Fire Agate (Chalcedony)
- ·Fluorite
- ·Gold
- ·Goshenite (Beryl)
- ·Grossular Garnet (Tsavorite Garnet)
- ·Gypsum
- ·Heliodor ( Beryl )
- ·Hematite
- ·Hessonite (Grossular Garnet)
- ·Imperial Topaz
- ·Indicolite ( Tourmaline)
- ·Iolite
- ·Ivory
- ·Jadeite
- ·Jasper (Chalcedony)
- ·Jet
- ·Kornerupine
- ·Kunzite
- ·Kyanite
- ·Labradorite
- ·Lapis Lazuli ( Lazurite)
- ·Lazulite
- ·Malachite
- ·Meerschaum
- ·Microcline
- ·Milky Quartz
- ·Moonstone
- ·Morganite ( Beryl)
- ·Nephrite ( Jade)
- ·Obsidian
- ·Oligoclase
- ·Onyx
- ·Opal
- ·Orthoclase
- ·Padparasha ( Corundum)
- ·Pearl
- ·Peridot
- ·Pink Topaz ( Mercury Myst Vapor )
- ·Plasma ( Chalcedony)
- ·Prase ( Chalcedony )
- ·Prehnite
- ·Pyrite
- ·Pyrope ( Garnet )
- ·Rock Crystal ( Quartz )
- ·Rubellite ( Tourmaline)
- ·Ruby ( Corundum)
- ·Sapphire ( Corundum)
- ·Sardonyx ( Chalcedony)
- ·Scapolite
- ·Schorl (Tourmaline)
- ·Shell
- ·Spessarite (Garnet)
- ·Sphalerite
- ·Spinel
- ·Spodumene (Hiddenite - Triphane)
- ·Tanzanite (Zoisite)
- ·Tektites ( Moldavite )
- ·Tiger Eye
- ·Titanite (Sphene)
- ·Topaz
- ·Turquoise
- ·YAG (Garnet)
- ·Zircon